Complicações do tratamento de um paciente com Pênfigo Vulgar com difícil manejo clínico: relato de caso
##plugins.themes.bootstrap3.article.main##
Resumo
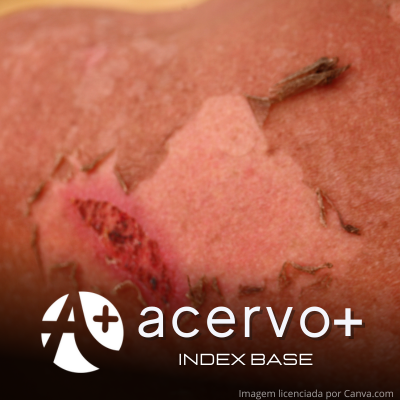
Objetivo: Relatar o caso de um paciente com diagnóstico de Pênfigo Vulgar (PV), de difícil controle clínico, que evoluiu com complicações graves ao tratamento convencional e melhora após uso de Rituximabe (RTX). Detalhamento do caso: Trata-se de um estudo observacional descritivo, do tipo relato de caso, sobre um paciente do sexo masculino, 42 anos, acompanhado no ambulatório de dermatologia do Hospital Universitário Lauro Wanderley da Universidade Federal da Paraíba entre os anos de 2020 e 2022. O paciente apresentava lesões bolhosas e exulceradas disseminadas em pele e mucosas, tendo o diagnóstico de PV confirmado por estudo histopatológico de fragmento de pele. Foi iniciado terapêutica com corticosteroide (CTC) sistêmico associado com Azatioprina, porém houve refratariedade ao tratamento, além de complicações como infecção bacteriana secundária e múltiplos episódios de hemorragia digestiva alta. Após avaliação laboratorial, o paciente foi submetido à infusão de RTX, com excelente resposta clínica. Considerações finais: O tratamento convencional do PV pode evoluir com complicações graves, as quais prolongam o tempo de internação e aumentam o risco de mortalidade e sequelas permanentes. Logo, o manejo com o paciente grave deve ser o mais eficaz, precoce e seguro possível. No caso apresentado, a associação do RTX ao tratamento resultou na melhora clínica do PV, possibilitando, assim, o controle adequado das complicações.
##plugins.themes.bootstrap3.article.details##
Copyright © | Todos os direitos reservados.
A revista detém os direitos autorais exclusivos de publicação deste artigo nos termos da lei 9610/98.
Reprodução parcial
É livre o uso de partes do texto, figuras e questionário do artigo, sendo obrigatória a citação dos autores e revista.
Reprodução total
É expressamente proibida, devendo ser autorizada pela revista.
Referências
2. AMAGAI M, et al. Usefulness of enzyme-linked immunosorbent assay using recombinant desmogleins 1 and 3 for serodiagnosis of pemphigus. British Journal of Dermatology, 1999; 40(2): 351-7.
3. BARCELOS V, et al. Epidemiological and clinical study of cases of endemic pemphigus foliaceus and pemphigus vulgaris in a reference center in the state of Minas Gerais, Brazil. Anais Brasileiros de Dermatologia, 2024; 99(1): 43-52.
4. BROEN JCA, VAN LAAR JM. Mycophenolate mofetil, azathioprine and tacrolimus: mechanisms in rheumatology. Nat Rev Rheumatol., 2020; 16(3): 167-178.
5. CARSON PJ, et al. Influence of treatment on the clinical course of pemphigus vulgaris. Journal of the American Academy of Dermatology, 1996; 34(4): 645-52.
6. CHAMS-DAVATCHI C, et al. Pemphigus: Analysis of 1209 cases. International Journal of Dermatology, 2005; 44: 470-76.
7. CHEN DM, et al. Rituximab is an effective treatment in patients with pemphigus vulgaris and demonstrates a steroid-sparing effect. Br J Dermatol., 2020; 182(5): 1111-19.
8. DAVARMANESH M, et al. Oral Pemphigus Vulgaris Treatment with Corticosteroids and Azathioprine: A Long-Term Study in Shiraz, Iran. Evid Based Complement Alternat Med., 2022; 2022: 7583691.
9. DOMÍNGUEZ-FRANCO A, et al. Pain Management in Patients with Severe Pemphigus Vulgaris. Journal of Pain & Palliative Care Pharmacotherapy, 2021; 35(4): 278-82.
10. GAGNIER J. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Global Advances in Health and Medicine, 2013; 5(5): 38-43, 2013.
11. GONÇALVES GAP, et al. Incidence of pemphigus vulgaris exceeds that of pemphigus foliaceus in a region where pemphigus foliaceus is endemic: Analysis of a 21-year historical series. Anais Brasileiros de Dermatologia, 2010; 86(6): 1109-12.
12. GONÇALVES LM, et al. Clinical evaluation of oral lesions associated with dermatologic diseases. Anais Brasileiros de Dermatologia, 2010; 84(6): 585-92, 2010.
13. HARMAN KE, et al. A study of desmoglein 1 autoantibodies in pemphigus vulgaris: racial differences in frequency and the association with a more severe phenotype. Br J Dermatol., 2000; 143(2): 343–8.
14. HERROD PJ, et al. Triple-ostomy: management of perforations to the second part of the duodenum in patients unfit for definitive surgery. The Annals of the Royal College of Surgeons of England, 2011; 93: 122–24.
15. JOLY P, LITROWSKI N. Pemphigus group (vulgaris, vegetans, foliaceus, herpetiformis, brasiliensis). Clinics in Dermatology, 2011; 29(4): 432-436.
16. JOLY P, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. The Lancet, 2017; 389(100830): 2031-40.
17. JOLY P, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med., 2007; 357(6): 545-52.
18. KERSHENOVICH R, et al. Diagnosis and classification of pemphigus and bullous pemphigoid. Autoimmunity Reviews, 2014; 13(4-5): 477–48.
19. KRIDIN K. Pemphigus group: overview, epidemiology, mortality, and comorbidities. Immunologic Research, 2018; 66(2): 255-70.
20. KORMANN. New and emerging therapies in the treatment of blistering diseases. Dermatologic Clinics, 2000; 18(1): 127-37.
21. MABTHERA: Solução para diluição para infusão. Farm. Resp: Liana Gomes de Oliveira. São Paulo: Roche Químicos e Farmacêuticos S.A., 2023. Bula de remédio.
22. MENTINK LF, et al. Randomized controlled trial of adjuvant oral dexamethasone pulse therapy in pemphigus vulgaris: PEMPULS trial. Arch Dermatol., 2006; 142(5): 570-6.
23. NARUM S, et al. Corticosteroids and risk of gastrointestinal bleeding: a systematic review and meta-analysis. BMJ Open., 2014 May 15; 4(5): e004587.
24. NGUYEN VT, et al. Pemphigus vulgaris IgG and methylprednisolone exhibit reciprocal effects on keratinocytes. J Biol Chem., 2004; 279(3): 2135-46.
25. NIHO K, et al. A case of bleeding duodenal ulcer with pemphigus vulgaris during steroid therapy. Clinical Journal of Gastroenterology, 2014; 7(3): 223–27.
26. OHATA C, et al. Locations of acantholysis in pemphigus vulgaris and pemphigus foliaceus. Journal of Cutaneous Pathology, 2014; 41(11): 880–89.
27. POLLMANN RP, et al. Pemphigus: A Comprehensive Review on Pathogenesis, Clinical Presentation and Novel Therapeutic Approaches. Clinical Reviews in Allergy & Immunology, 2018; 51(1): 1-25.
28. SÁNCHEZ-PÉREZ J, GARCÍA-DÍEZ A. [Pemphigus]. Actas Dermosifiliogr., 2005; 96(6): 329-56.
29. SHAH AA, et al. Development of a Disease Registry for Autoimmune Bullous Diseases: Initial Analysis of the Pemphigus Vulgaris Subset. Acta Dermato-Venereologica, 2015; 95(1): 86-90.
30. SINHAA A, et al. Pemphigus vulgaris: approach to treatment. European Journal of Dermatology, 2015; 25(2): 103-13.
31. SNASTI L, et al. Treatment of Pemphigus Vulgaris and Foliaceus with Adjuvant Rituximab Compared to Immunosuppression Alone: Real-Life Experience. Dermatology, 2020; 237(2): 179-84.
32. SPINDLER V, WASCHKE J. Pemphigus - A Disease of Desmosome Dysfunction Caused by Multiple Mechanisms. Frontiers in Immunology, 2018; 9: 136.
33. TAGEJA N, et al. Upper gastrointestinal bleeding in a patient with a history of peptic ulcer disease: don't presume the diagnosis. South Med J., 2010 Feb; 103(2): 181-2.
34. WERTH V. Treatment of Pemphigus Vulgaris with Brief, High-Dose Intravenous Glucocorticoids. JAMA Dermatology, 1996; 132(12): 1435-39.
35. YALEM A, et al. The Importance of Patient-Focused Drug Development in Pemphigus and Pemphigoid. Journal of Investigative Dermatology, 2023; 143(10): 1868-71.